
June 27, 2018
The advancement of medical systems has made hospitals and other medical facilities into places that are expected to be safe. By applying the life-saving skills of the staff and utilizing reliable and powerful equipment, these facilities symbolize the value of human life and health.
But, as technology improves, this presents new complexities and hazards to the system. Therefore, medical electrical equipment must be compliant with applicable standards to protect individuals within medical facilities from potential harm.
In 1976, the Food & Drug Administration (FDA) was officially entitled to oversee medical devices based on the Federal Food, Drug and Cosmetic Act.
U.S. President Gerald Ford, who signed the legislation, stated: "Medical devices support and lengthen life when they are well designed, well made and properly used. But, when they are poorly designed, poorly made, and improperly used, they can threaten and impair life." His words still resonate with the FDA today.
The FDA believes that safe and effective medical devices are not risk-free. Therefore, based on how probable a risk is, and how severe the potential harm is to the user/patient -- and after undergoing rigorous risk assessment and mitigation processes - medical devices are categorized into 1 of 3 classes.

According to the IEC-60601-1 standard, a medical device must be designed to be Single Fault Safe.
According to IEC-60601-1: "Single Fault Safe is defined as a characteristic of medical electrical equipment, or it's parts, whereby it remains free of unacceptable risk during its expected service life under Single Fault Condition; a condition in which a single means for reducing a risk is defective or a single abnormal condition is present."
In other words, if one failure happens in either the device itself (such as a failed component) or external to the device (such as a power failure to the building), the patient or the operator should remain unharmed.
A Single Fault Condition can occur at any time. It is when either:
A single means for reducing a risk fails
OR
A single abnormal condition occurs
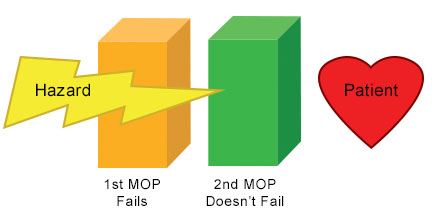
In the illustration above, the medical device remains Single Fault Safe after the Single Fault condition occurs; the patient remains unharmed
Leakage current is the current that flows from a point of contact, through the conductor making contact, then to the ground. Electricity always tries to find a way to the ground (earth).
For example: When a person comes in contact with a product that is operating, leakage current is the amount of current that flows from the point of the person's contact with the product, through the person's body and then to the ground.
According to IEC-60601-1, based on how a person might come in contact with the product, leakage current can be defined as:
Some electrical equipment uses a two-prong power cord to power/charge a device for operation. By touching these products, some level of leakage current will always flow through one's body to the ground. Therefore, the accessible parts need to be separated from the live parts by two layers of insulation. This is accomplished with either Basic + Supplemental Insulation or Reinforced Insulation.
In portable devices, or products with three-prong power cables, one layer of insulation (basic insulation) may suffice, due to one of the prongs' role in grounding the leakage current.

Medical devices are categorized into one of three classes and within each class, a product can have applied parts from any type: "B", "BF", and "CF".
Based on the class & type, IEC 60601-1 specifies an acceptable level of leakage current towards the patient for user safety. This level is defined in a way that always assures there are two layers of protection for patients and operators to ensure that design elements are always within safe limits to prevent leakage current harm.
The following table shows examples of leakage current limitations for a medical device.
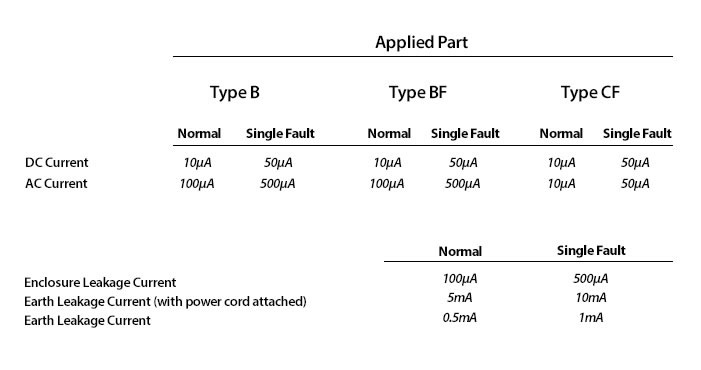
Adherence to these limits is a crucial element in the operation of electrical medical devices as it protects us all from hazards.
Due to environmental phenomena, static electricity can build-up on an object and will not be discharged until a second object with a different level of static charge comes into close proximity.
The discharge occurs in the form of an electric shock when the objects are close enough to each other. The shock is caused by the object with the higher charge discharging electricity to the object with a lower charge.
Hospital equipment (machines, devices, etc.), like most other electrical devices, are vulnerable to static electric shocks imposed by the environment. This can cause the equipment to short circuit, cease operation, or malfunction, which can cause harm to the patient and/or user.
A shock can also be extremely dangerous if it happens in an oxygen-enriched environment.

A Medical Device must consist of three main parts; an enclosure, insulation, and applied parts.
The main role of insulation is to keep the applied parts safe from any unwanted environment/external distortion or current flowing towards it.
The applied part is that which is in direct contact with the patient, so special care in designing for safety is needed here to mitigate any threats to the patient's life.
To avoid any static charge build-up on an object, another solution is to consciously discharge through a conductor to the earth.
In well-designed medical equipment, environmental static charge drains from the ground connector to the earth through a grounded wire.
This wire must be installed properly and cannot be disrupted in any way or else all static build-up will seek an alternative path to discharge, possibly through the patient and/or user's body if they link the device to the ground.
The goal of every Medical Device is to restore or improve human health while providing freedom from harm.
Please contact us for more information about Medical Device Electrical Safety, or any other Medical Electrical design work.
© 2019 Koven Technology Canada Inc.