
April 10, 2018
Creating your Design Input Specification (DIS) should be the first stage of any product design process.
It is a vital tool your design team will use as the framework for the design and development of your medical device, and to comply with regulatory requirements.
In the simplest terms, your Design Input Specification will establish the medical device requirements relating to function, safety, design, and performance. The DIS should be established independent of any specific product solution, therefore allowing multiple concept possibilities in the ideation phase which follows:
It is the collection of all design inputs for your device. Your design inputs cannot be incomplete, ambiguous, or conflict with each other.
There are many sources of design inputs, but a key input is the needs of the user (and/or patient), including risk assessment and human factors considerations.
Well defined user needs breathe life into your product design. They are the reasons behind each shape and function of your medical device and they represent the voice of the consumer.
To make them useful for designers, they must be expressed in metric form.
They are the quantitative and measurable criteria that your product will comply with. They must contain a metric, a unit, and a value to be measurable, testable, unambiguous, and traceable.
SO HOW DO WE TRANSLATE USER NEEDS INTO SPECIFICATIONS? The task of applying specs to something that feels immeasurable may feel intimidating. But we're innovators -- we make it work!
For this guide, examples were drawn from the development file of a Leg Stent Deployment System (LSDS) that was designed by our team.
Who is the customer? What are their needs?
Below are steps you should take and some questions you should ask to determine user needs.
Following are five different examples drawn from observations, surveys, interviews, and competitor analyses.
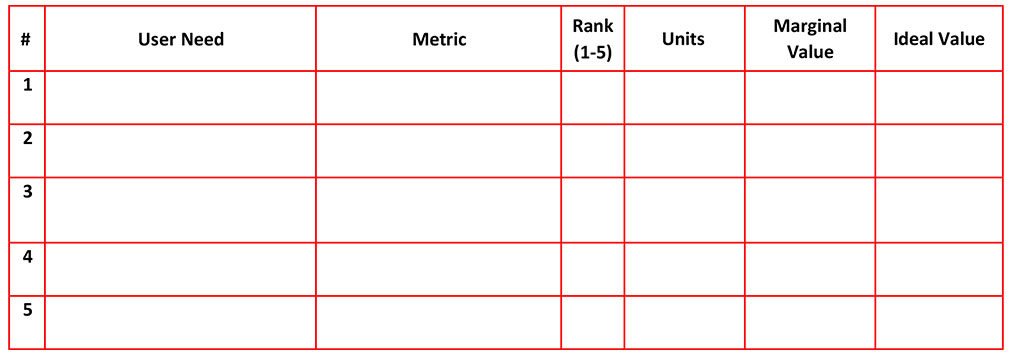
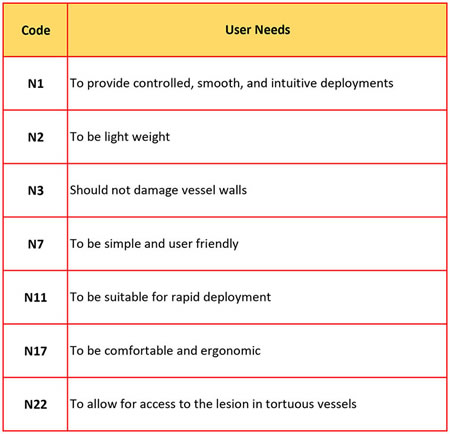
A sample of the LSDS device USER NEEDS are:
Once you know WHAT the user needs are, you must then establish HOW the device will meet them.
This is the trickiest part of your design input specification development.
Karl T. Ulrick & Steven D. Eppinger provide an excellent resource for product specifications in their book: "Product Design & Development, 5th Ed." (Chapter 6).
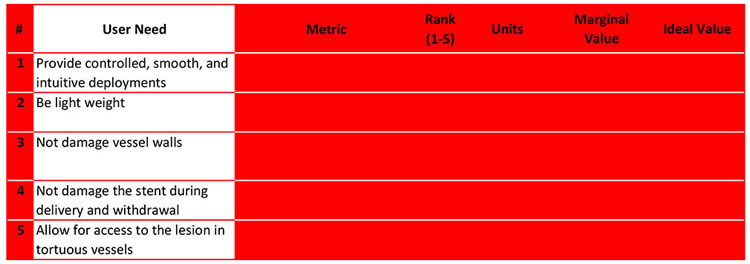
You want to brainstorm a way to objectively measure each of your user needs. Strive for dependent variables that allow the most freedom for your design team.
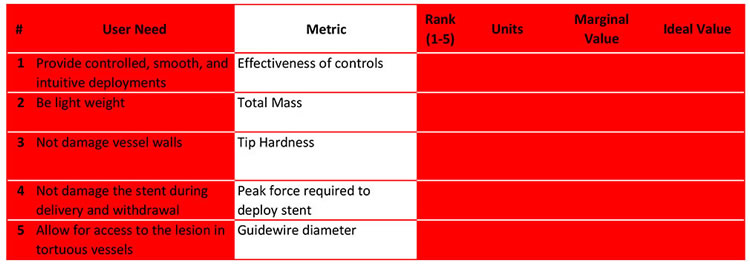
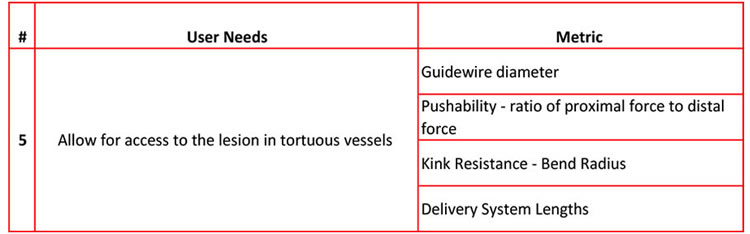
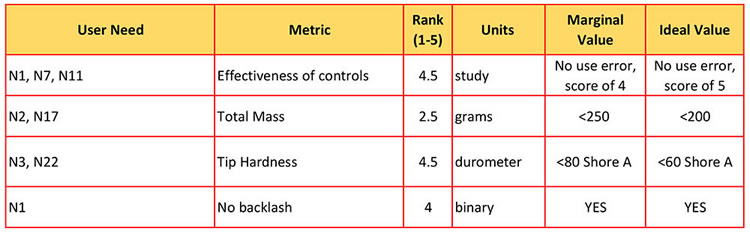
As you will find, each user need can be measured by multiple metrics. For example, user need #5 can be measured by 4 different metrics and possibly more.
Alternately, metrics can measure multiple different user needs.
To prepare for designers, we TURN THESE METRICS INTO SPECIFICATIONS BY ADDING UNITS & VALUES.
The rank determines the level of importance we place on our units and values. Establishing these rankings can take some research.
Here's how we ranked our items:
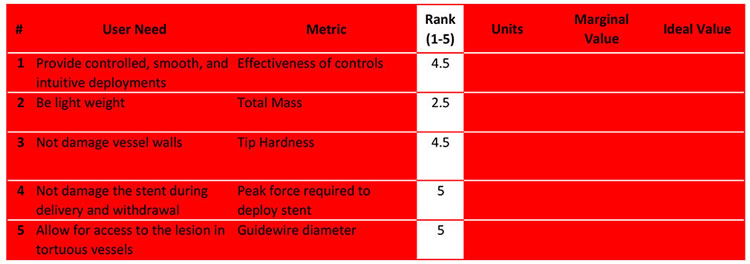
We determined that the weight (#2) of the finished product was of mid-importance, thus won't be a top concern in the design process.
Being able to access the lesion (#5) is very important (it is the primary purpose of the stent deployment system). And, the stent must not get damaged (#4) or break. This would cause the limb-saving procedure to fail and possibly embed pieces of metal in the vascular system.
Therefore, items #4 and #5 get a top rank of 5 and to emphasize that they will receive the greatest focus throughout the design process.
This is quite easy for some metrics and may seem impossible for others.
Keep in mind, nearly everything in this world can be calculated using universal standards of measurement. We just have to the find the right one.
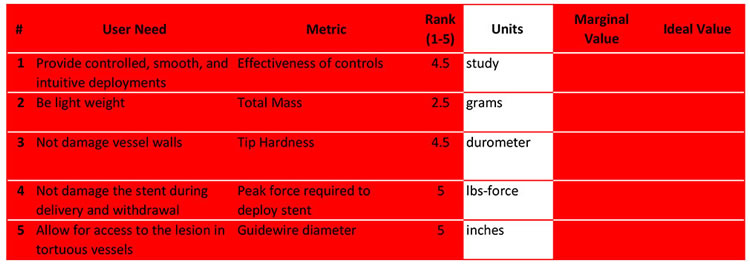
We decided the best way to measure control effectiveness (#1) would be to conduct a study in which participants will use the device and complete a survey or questionnaire. The results can be translated to a numerical score.
There are standard units of measurement that are followed for certain metrics, like durometer, pounds, millimeters, etc. When a yes or no is required, we list the unit as binary.
Once established, you may also need to determine the scale or precision; mm or in? Seconds or nanoseconds? Lbs or kg? Months or weeks? Etc. You can research similar products to see what the standard units are.
It is important to set limits and to have a goal.
Marginal value is the unit amount limit we choose for our user need metrics.
Ideal value is the unit amount that we strive to achieve
This clearly defines our acceptance criteria, while providing the design team with "room to move".
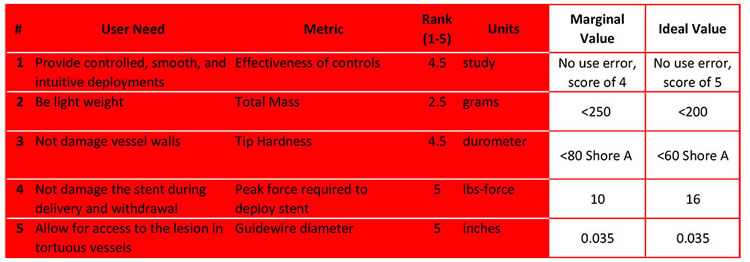
For item #2, the ideal total mass is less than 200g. If the ideal doesn't work out, we know that it can't be more than 250g, which is our margin.
For item #5, it is imperative that the guidewire diameter is exactly 0.035 inches. Guidewires with this diameter are the standard product used in the radiology lab for the procedure, and the deployment catheter cannot be used without it.
The values you choose are very important; Your finished product must meet these values. They can be the difference between success and failure.
AND THERE YOU HAVE IT! SPECIFICATIONS DERIVED FROM YOUR USER NEEDS.
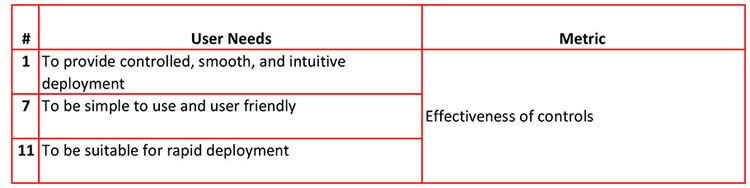
I mentioned that there was an easier way to organize multiple user needs and metrics. Once you determine all user needs and matched up the metrics, give each user need a code name.
User need #7 gets coded N7. the user needs N1, N7 and N11 can all be measured using the "effectiveness of controls" metric.
For more ease, you should have two tables; one defining all the user needs and their code names, and a second one that lines up all the user needs with the metrics.
To further organize, you can assign a code name to each metric row, like M1 (Metric #1)
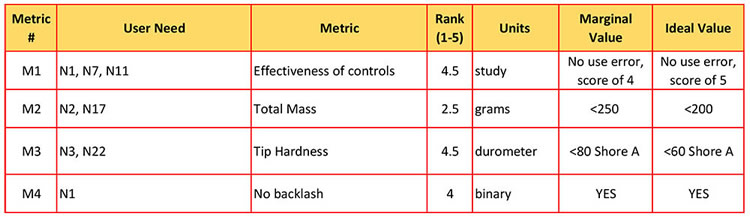
Now your specifications have traceability! (Metrics tied to a user need)
See how it looks clean and organized in these final tables?
It will be hard to get confused or lost with your user needs and metrics being this highly organized. In addition, you provide the required traceability necessary to comply with FDA design control regulations.
If you enjoyed this how-to guide or know of anyone who would find it useful, please feel free to share on any social media platform.
If you have questions, please contact me on LinkedIn or through our contact page.

© 2019 Koven Technology Canada Inc.